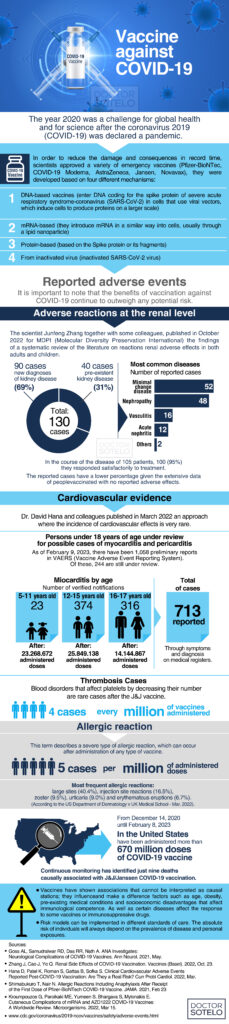
In this week’s blog, we will discuss the COVID-19 vaccine, the basis for its development, the booster schedules, some adverse effects, statistical mortality rates, and finally, the vaccine itself.
It is no secret that 2020 was challenging for global health and science after the 2019 coronavirus (COVID-19) was declared a pandemic. Scientists developed and approved several emergency vaccines to mitigate its ravages and consequences in record time: Pfizer-BioNTec, Moderna, AstraZeneca, Janssen, and Novavax.
As more patients access the 2019 coronavirus disease vaccines (COVID-19), different specialists are faced with questions about possible complications, benefits, and the ideal timing of vaccination and their boosters.
As a man of science grounded in evidence, I want to highlight that vaccines have demonstrated associations that cannot have a causal interpretation; factors such as age, obesity, pre-existing medical conditions, and socioeconomic disadvantages influence and make a difference. These characteristics stand out among those best known to impact immune competence, just as certain diseases affect the response to some vaccines or drugs that alter our immune system.
The absolute risk to individuals will always depend on the prevalence of COVID-19 and personal exposure. We are following a vaccination protocol until we reach a period that reflects factual statistical data that can be conclusive regarding adverse effects or mortality due to the vaccine. What is certain is that the vaccine prevents complications in people with the virus and the rate of contagion among people.